

Africa’s healthtech industry is facing stricter regulations in 2025. Governments are formalizing the sector to ensure patient safety, data privacy, and ethical operations. For startups, compliance is now a must to avoid legal trouble and access national healthcare systems.
Key Updates:
- Nigeria’s Digital Health Services Bill (March 2025): Requires licenses for telemedicine and AI platforms. Penalties include $3,350 fines and up to 5 years in prison.
- Data Privacy: Over 50% of African nations now have data protection laws. Cross-border data sharing is being standardized under the Africa CDC framework.
- Interoperability Standards: Startups must adopt HL7/FHIR for seamless integration with healthcare systems.
- Opportunities: Nigeria’s Startup Act offers tax breaks and funding for compliant companies.
Takeaway: To thrive in Africa’s evolving healthtech space, founders must prioritize licensing, data governance, and interoperability. Early compliance can unlock growth opportunities while safeguarding operations.
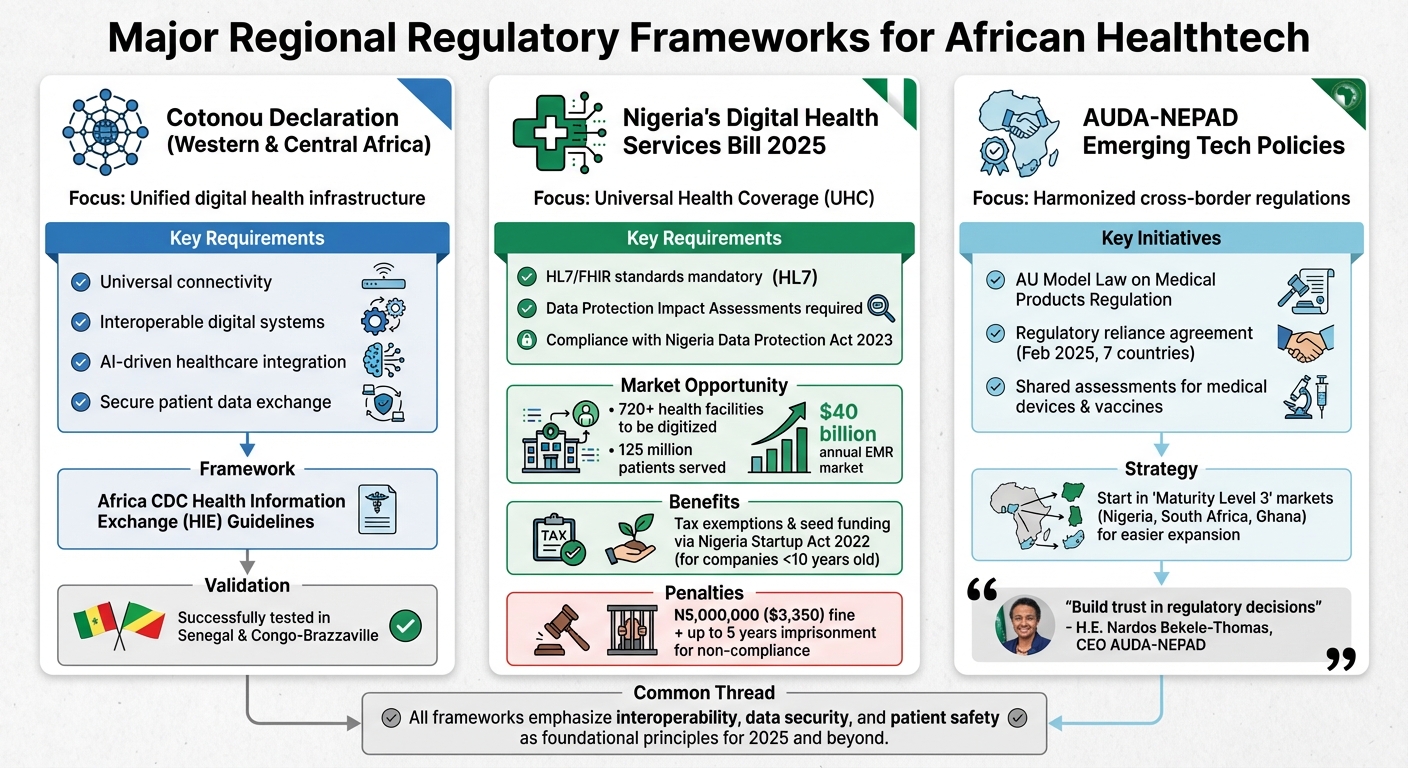
Major Regional Regulatory Frameworks

Africa Healthtech Regulatory Frameworks 2025: Key Requirements by Region
Healthtech founders in Africa need to navigate three major regional frameworks that set the standards for digital health operations. These frameworks outline the specific requirements startups must incorporate to align with regional regulations.
Cotonou Declaration on Digital Transformation
The Cotonou Declaration focuses on creating a unified digital health infrastructure across Western and Central Africa. It emphasizes universal connectivity, interoperable digital systems, and the integration of AI-driven healthcare solutions. This framework is closely tied to the Africa CDC’s Health Information Exchange (HIE) guidelines, which were successfully validated in Senegal and Congo-Brazzaville to enable seamless cross-border data sharing. Startups operating in this region must ensure their platforms support secure and standardized patient data exchange.
Nigeria’s Healthtech Guidelines and UHC Efforts
Nigeria’s Digital Health Services Bill 2025 is a key part of the country’s strategy to achieve Universal Health Coverage (UHC), as outlined in its National Health Policy. This legislation expands access to regulated digital healthcare services. Startups are required to adopt HL7/FHIR standards and comply with the Nigeria Data Protection Act 2023, which mandates the completion of Data Protection Impact Assessments. Through the Nigeria Digital in Health Initiative (NDHI), the government aims to digitize over 720 health facilities and serve approximately 125 million patients. This presents a substantial market opportunity, with the African electronic medical records (EMR) market valued at an estimated $40 billion annually.
Additionally, companies less than 10 years old can benefit from the Nigeria Startup Act 2022, which offers tax exemptions and seed funding to officially recognized startups.
AUDA-NEPAD‘s Emerging Tech Policies
AUDA-NEPAD is focused on harmonizing regulatory frameworks across Africa through initiatives like the AU Model Law on Medical Products Regulation. In February 2025, seven countries signed a regulatory reliance agreement to share assessments of medical devices and vaccines, enabling faster multi-country approvals. This framework complements existing ethical oversight and aims to build a more collaborative regulatory environment. For instance, minoHealth AI Labs in Ghana demonstrates how using local data can improve AI diagnostics while keeping costs manageable.
H.E. Nardos Bekele-Thomas, CEO of AUDA-NEPAD, remarked:
“For Africa to achieve a harmonized regulatory system, we must build trust in one another’s regulatory decisions. This agreement is a step forward in fostering collaboration and ensuring that safe and effective medical products reach our populations faster.”
Startups looking to expand across Africa should consider starting in “Maturity Level 3” markets like Nigeria, South Africa, or Ghana. These countries’ regulatory systems can simplify the process of entering other signatory markets under the new reliance agreement.
Data Governance and Privacy Standards
In Africa, health data is considered a critical resource that demands strict protection. By the end of 2024, over half of African countries had enacted data protection laws, with momentum building toward unified standards across the continent. For healthtech entrepreneurs, this means navigating not just local regulations but also emerging regional frameworks that will influence cross-border operations.
Africa CDC‘s Data Governance Initiatives

Africa CDC has introduced three key tools to help healthtech startups manage patient data responsibly. The Model Law on Health Data Governance serves as a foundation for national legislation across member states, emphasizing equity and rights-based principles. This law was developed with input from more than 1,000 global stakeholders.
In July 2025, Africa CDC unveiled the Continental Health Data Governance Framework, aimed at creating a cohesive African stance on health data management and enabling secure cross-border data sharing. Scheduled for endorsement at the African Union Summit in February 2026, this framework could mark the first unified agreement on cross-border health data sharing. Dr. Martha Terefe, Deputy Chief of Staff at Africa CDC, highlighted its importance:
“Health data is powerful. When governed well, it can strengthen health systems, improve health outcomes, support real-time decision-making, and close equity gaps.”
The AU Health Information Exchange (HIE) Guidelines complement this framework by setting technical standards for health data sharing, focusing on interoperability and secure exchanges. These guidelines align with the Malabo Convention on Cybersecurity and Personal Data Protection, which is the cornerstone for data security. Healthtech startups can use the Africa CDC assessment tool to ensure their platforms meet these standards.
Startups must also establish robust consent and security measures to comply with these frameworks.
Consent, Ownership, and Security Protocols
In many African countries, health and genetic data are subject to stricter regulations compared to general personal data. A 2024 analysis of 37 data protection frameworks confirmed these heightened standards. Startups need to ensure their systems uphold key data subject rights, such as the rights to be informed, access data, request corrections, erase data, transfer data, and object to processing.
Static, one-time consent models are no longer sufficient. Modern platforms now emphasize real-time patient engagement and dynamic rights management. Jean Philbert Nsengimana, Chief Digital Advisor at Africa CDC, described this shift:
“In Africa, we are not just addressing the challenges of governance but seizing the opportunity to build frameworks that prioritize equity, trust, and ethical use.”
For cross-border data transfers, startups can rely on one of four legal mechanisms: adequacy agreements, standard contractual clauses, binding corporate rules, or explicit informed consent. Since adequacy assessments between African nations are still inconsistent, many startups use standardized contractual clauses and Trusted Research Environments (TREs) – secure platforms that allow data analysis without moving raw datasets across borders.
The “Five Safes” framework offers a practical compliance guide, focusing on safe projects, safe people, safe data, safe settings, and safe outputs. Startups should also implement a “safe data flow module” that includes adequacy checks, pre-approved contractual clauses, and mandatory staff training in data protection.
| Core Principle | Requirement for Healthtech Startups |
|---|---|
| Data Minimality | Process only the personal data absolutely necessary for your purpose |
| Purpose Specification | Collect data for a specific, clearly defined health-related purpose |
| Storage Limitation | Retain data only as long as needed for its intended purpose |
| Accountability | Demonstrate compliance with all principles; you’re responsible as the controller |
Incorporating these principles into compliance strategies is essential for keeping up with evolving healthtech regulations.
With a 41% increase in disease outbreaks and only 30% of health systems digitized, the need for strong data governance has never been more pressing.
How to Navigate Regulatory Complexity
Africa’s healthtech sector operates under 37 distinct data protection frameworks, each with its own rules for consent, data storage, and cross-border transfers. For founders expanding across multiple countries, this patchwork of regulations can pose operational hurdles that demand careful planning and robust compliance strategies.
Building Compliance Processes
Start by appointing a compliance lead or a Data Protection Officer (DPO). In countries like Gabon, Senegal, and Lesotho, legal mandates require advisory committees for processing research data. Your compliance team should oversee these relationships and stay updated on regulatory changes in your target markets.
Engage early with regulators. Collaborate with Ministries of Health during the technical development phase, well before your product launch.
For cross-border operations, establish a “safe data flow module” that includes adequacy assessments, pre-approved Standard Contractual Clauses (SCCs), and mandatory staff training on data protection. In regions like Madagascar, Mali, South Africa, and Zambia, SCCs are essential for legal data transfers when “adequacy” status isn’t granted. Trusted Research Environments (TREs) can also be a practical solution, allowing data analysis without physically transferring personal data across borders.
Transparency is key – display your professional registration numbers prominently on all platforms. In Nigeria, failing to operate with proper licensing can result in penalties of at least N5,000,000 (about $3,350) and up to five years in prison. Additionally, secure professional indemnity insurance to protect against liabilities from medical or technical failures. If you’re using foreign technology or intellectual property, register it with national offices like Nigeria’s NOTAP.
Keep an eye on the Continental Health Data Governance Framework, which is expected to be endorsed at the African Union (AU) Summit in February 2026. This framework aims to streamline cross-border compliance, making it easier to navigate the regulatory landscape.
Once your compliance systems are in place, ensure your technical infrastructure supports seamless integration by adopting widely recognized interoperability standards.
Using Interoperability Standards
Following the African Union Health Information Exchange (AU HIE) guidelines provides a clear path for technical development across the continent’s five regions. These guidelines address the lack of standardized systems that currently hinder effective data sharing.
Adopt HL7/FHIR standards early to simplify compliance. Nigeria’s Digital Health Services Bill 2025, for example, requires all digital health platforms to be compatible with the country’s electronic health record (EHR) infrastructure. Building this compatibility from the outset avoids the costly process of redesigning systems when entering new markets with strict integration demands.
The AU HIE guidelines focus on three main areas: policy directions, standards, and implementation use cases. Developed by a task force of 24 experts, they aim to create a cohesive framework for data sharing across African regions. By aligning with these standards, you can avoid the need to design separate systems for each country.
Incorporate privacy-by-design principles and conduct Data Protection Impact Assessments (DPIAs) for every data processing activity. This approach ensures compliance with local laws like the Nigeria Data Protection Act, as well as emerging regional frameworks. As Ridwan Oloyede from Tech Hive Advisory Africa points out:
“The framework risks becoming quickly outdated as technology continues to evolve… A more resilient approach would be to regulate the act of providing healthcare.”
Finally, consider partnering with locally licensed entities in your target markets. These partnerships can help you navigate licensing requirements while building trust with local regulators. At the same time, your interoperable technical systems will ensure smooth operations across borders.
sbb-itb-dd089af
Staying on top of compliance in Africa’s dynamic regulatory environment requires more than manual tracking – it demands the use of advanced tools and platforms. For instance, RegDesk offers a proactive approach by monitoring and analyzing regulatory changes for medical device and biotech companies. This shifts compliance efforts from reactive to forward-thinking, ensuring companies stay ahead of policy updates before they take effect. As RegDesk explains:
“Compliance is not just about meeting standards – it’s about setting them.”
Other platforms also play a key role in supporting innovation. For startups working on cutting-edge technologies like AI-driven diagnostics or genomic medicine, the Health Tech Platform – a joint initiative by AFIDEP and AUDA-NEPAD – provides essential technical guidance. This platform helps founders navigate the regulatory approval process for emerging technologies, including AI algorithms and drone-based medical delivery systems.
In addition to regulatory updates and technical support, certain platforms tackle specific challenges like electronic health records (EHR) compliance and licensing. Verisys addresses these needs by offering real-time, blockchain-verified data to prevent fraud and ensure compliance with licensing requirements. Their cloud-based system incorporates multi-factor authentication and encryption, aligning with HIPAA standards and African data privacy laws.
To further streamline compliance efforts, the Africa CDC Health Data Governance Legislative and Regulatory Assessment Tool and its Technical Implementation Guide provide actionable insights. These resources help identify compliance gaps and translate the Model Law into practical operational steps. Developed under the Africa CDC Flagship Initiative, these tools reflect contributions from nearly 1,000 stakeholders in shaping data governance standards across the continent.
Finally, IQVIA assists healthtech companies by evaluating market readiness for digital health solutions and offering guidance on regulatory pathways. By prioritizing investments in markets with high potential, companies can achieve efficiency gains of up to 15%. These tools and resources are invaluable for healthtech innovators, helping them stay compliant while maintaining a competitive edge in an ever-changing regulatory landscape.
Conclusion
Key Takeaways for Healthtech Founders
The regulatory landscape for healthtech in Africa has undergone a significant transformation. In Nigeria, operating without proper authorization now carries severe penalties, including hefty fines and imprisonment. Similarly, South Africa has introduced stringent certification requirements. What was once a loosely defined framework has evolved into a structured system with clear licensing regimes and serious consequences for non-compliance.
To stay ahead, healthtech founders must prioritize licensing, data governance, and technical interoperability. Securing sector-specific licenses – whether from Nigeria’s Federal Ministry of Health or South Africa’s SAHPRA – is no longer optional. Additionally, implementing privacy-by-design principles, conducting Data Protection Impact Assessments (DPIAs), and adopting standardized data formats like HL7/FHIR are critical steps for ensuring compliance and operational success.
This shift also presents an opportunity. The Nigeria Startup Act 2022, for example, provides tax breaks and financial incentives for startups that align with its requirements. Early compliance not only meets legal obligations but also creates a competitive edge in the market. As Ridwan Oloyede of Tech Hive Advisory explains:
“The success of this bill will therefore depend not only on the capacity of the Federal Ministry of Health but also on its ability to develop implementing regulations that are principles-based and outcome-focused, safeguarding patients without stifling the very innovation the bill seeks to promote”.
Compliance isn’t optional – it’s essential. Engaging with regulators early, securing indemnity insurance, and taking advantage of startup incentives can pave the way for long-term success. By embracing these changes, healthtech founders have the chance to build trust with patients and position themselves for sustainable growth in Africa’s evolving healthtech sector.
FAQs
What compliance requirements should African healthtech startups prepare for by 2025?
By 2025, healthtech startups across Africa will need to navigate a range of compliance requirements to operate legally and maintain a competitive edge. These include securing the necessary licenses from national health authorities, such as Nigeria’s Federal Ministry of Health, and ensuring their platforms meet technical standards and data privacy regulations. Features like consent management, encryption, and audit trails will be essential components of their systems.
On a broader scale, the Africa CDC’s Health Data Governance Framework will demand clear policies for managing data – covering everything from collection and storage to sharing and cross-border transfers. Meanwhile, the African Union Model Law on Medical Products Regulation will require startups to register their medical devices and health software, as well as conduct quality testing and implement post-market monitoring. Telemedicine services will face additional scrutiny, needing to comply with rules around professional certifications, patient consent processes, and tax obligations specific to the sector.
For those working with AI in healthcare, emerging regulations will emphasize ethical design, transparency in algorithms, and thorough impact assessments for AI-driven tools. By addressing these requirements – spanning licensing, data governance, product safety, and AI ethics – African healthtech startups can position themselves to thrive in a rapidly evolving regulatory environment.
How can healthtech startups comply with data privacy laws and manage cross-border data sharing?
Healthtech startups can navigate the complexities of African data privacy regulations and cross-border data sharing by adopting a compliance-by-design approach. This means embedding privacy principles into every aspect of their operations. For example, South Africa’s Protection of Personal Information Act (PoPIA) emphasizes key practices like obtaining explicit consent, limiting data collection to what’s absolutely necessary, encrypting sensitive information, and appointing a dedicated data protection officer to manage policies and compliance.
Beyond national laws, startups should also align with broader frameworks like the African Union’s Data Policy Framework and the upcoming Continental Health Data Governance Framework. These initiatives aim to create consistent standards for secure cross-border data sharing, including requirements for formal data-sharing agreements and certification processes.
To tackle country-specific rules, startups can rely on practical tools and strategies. For instance, they should familiarize themselves with local data residency laws in countries such as Kenya, Nigeria, and Rwanda, while ensuring they document lawful bases for all data transfers. Combining strong internal safeguards with standardized legal practices allows startups to protect patient privacy while scaling their operations across Africa and beyond.
What benefits does the Nigeria Startup Act provide for healthtech companies that comply with its requirements?
The Nigeria Startup Act of 2022 brings a host of advantages for healthtech companies that align with its registration and licensing requirements. By complying, startups can tap into tax benefits, including lower corporate income tax rates and exemptions on duties for imported medical technology equipment. On top of that, the Act simplifies the registration process with a centralized portal and provides formal certification, boosting trust and appeal among investors and business partners.
The Act also establishes the National Council for Digital Innovation and Entrepreneurship, which offers mentorship programs, helps startups secure public-sector contracts, and facilitates access to research grants. Another highlight is the Startup Investment Seed Fund, overseen by the Nigerian Sovereign Investment Authority. This fund connects eligible startups with seed capital and venture funding opportunities. Together, these initiatives aim to cut through regulatory red tape, attract investments, and support healthtech companies in scaling within Nigeria’s dynamic healthcare sector.
Related Blog Posts
Source link



